Therefore the valence electrons of the manganese ion Mn 3 are twelve. Use the Mendeleevs Periodic Table to figure out how many electrons Nitrogen N has in its valence shell.

Difference Between Valence And Core Electrons Compare The Difference Between Similar Terms
EHow was the number of electrons used for each resonance structure shown in Model 3.

. Nitrogen is in Group 5 so it has 5 outer shell electrons. The atomic number of nitrogen is 7 It is present in group 5 of the Periodic Table of Elements. How many valence electrons does each carbon atom have.
You guys are so stupid that say that it has 7. Sulfur has 6 valence electrons because it is in Group 16. The number of valence electrons is the number of electrons in the outer shell that the atom uses for bonding.
8aHow many valence electrons does one nitrogen atom have. The total number of electrons present in the valence shell of an atom are called valence electrons and there are a total of five electrons present in the valence shell of nitrogen 2s22p3. How many electrons does nitrogen gain or lose.
6 How many electrons does a carbon atom have in its outer energy level. Thus the Lewis structure is as in the first image below. To determine valence electrons add the outermost and orbitals.
See answer 1 A nitrogen atom has 5 valence electrons. You just studied 18 terms. Therefore it can accept 3 electrons to complete its octet.
11 How do you calculate shared electrons. Two inner shell core electrons in the 1s orbital and four valence. Valence electrons of nitrogen.
Atomic carbon has six electrons. B How many valence electrons does a nitrogen atom possess. BHow many valence electrons do three oxygen atoms have.
Thus the total number of valence electrons present in nitrogen atoms is five. Atomic nitrogen has 5 valence electrons and 4 valence orbitals 2s 2p x 2p y and 2p z. Nitrogen is in 5A of the PT and so has 5 valence electrons.
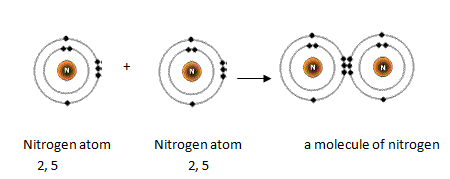
Since the last shell of a nitrogen ion has eight electrons the valence electrons of nitrogen ionN 3- are eight. 9Why is a resonance hybrid. Nitrogen is the 7th element so out of 7 electrons 2 go to the first shell and 5 to the second.
There are 2 ways to find the number of valence electrons in Nitrogen. Nitrogen being a non-metal has five electrons in its outermost shell and requires only three electrons to complete its octet and acquire stable electronic configuration of the nearest noble gas So nitrogen has a strong tendency to gain three electrons from the other electron donating. Valency of Nitrogen N is 3 as it has 5 electrons in its valence shell.
Remember dont confuse valence electrons with total electrons. It is present in group 5 of the Periodic Table of Elements. How many Valence Electrons does each atom have.
The energy levels in an atom go 2 8 18 32 50 72but the last energy shell is always 8 unless it is hydrogen or helium so in nitrogen the shells would be 2 5. How many valence electrons does Sulfur S Group 16 have. No Nitrogen has 5 Valence electrons.
People also ask how many valence electrons does SiF4 have. Nitrogen has 5 valence electrons because its electron configuration is. But typically a nitrogen atom gains 3 electrons to form the nitride ion N3.
A single chemical bond takes u one valence electron from each atom. It has 7 electrons distributed in 2 energy shells. 8 How many electrons do carbon and oxygen share.
In the Lewis structure there is a triple bond between the nitrogen atoms and a non-bonding pair of electrons on each. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements though. Thus nitrogen has five valence electrons.
7 How many electrons does one atom of carbon share to complete its valence shell. Sulfur has 14 total electrons based on its number of protons and then 6 of these are valence electrons. Again the manganese atom donates two electrons in 4s orbital and two electrons in 3d orbital to convert to manganese ion Mn 4.
CHow many valence electrons does one NO3 molecule have. C An atom has the electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 2. For SiF4 all the Si electrons are in bonds but each F atom has 6 electrons still available.
Up to 256 cash back a True or false. An elements number of valence electrons is the same as its atomic number. Nitrogen has 5 electrons in its n2 outer shell.
Mn 4e Mn 4. DHow many valence electrons does one NO3 ion have. 9 How many electrons can a carbon atom share quizlet.
10 Does carbon have 2 or 4 electrons. After determining how many valence electrons there are in NF3 place them around the central atom to complete the octetsThe NF3 Lewis structure has a total of 26 valence electronsNitrogen N is the least electronegative element and goes in the center of the Lewis structure for NF3. This electron configuration of manganese ion Mn 4 is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3.
The valence shell of aluminum has three electrons and per the octet rule these three electrons are lost resulting in just 10 electrons and 13 protons. Compound formation of nitrogen Nitrogen participates in the formation of bonds through its valence electrons. Now up your study game with Learn mode.

How Many Valence Electrons Does Nitrogen Have Quora

How Many Valence Electrons Does Nitrogen N Have
What Is The Number Of Valence Electrons In Nitrogen Socratic
0 Comments