The negative ions are formed by non-metals gaining one or more extra electrons. Electrical conductivity is the movement of charge in response to an electric field.

Ions Introduction To Chemistry
Metals and semiconductors the charge movement or current is due to electrons or holes and is described as electronic conductivity.
. Together they neutralize the compound. Ion any atom or group of atoms that bears one or more positive or negative electrical charges. It has six protons six electrons and six neutrons.
When one metal ion shares electrons with anthor metal ion it is called. Ions are formed by the addition of electrons to or the removal of electrons from neutral atoms or molecules or other ions. AnionIons that are negatively charged because they have more electrons than protons.
Metal ions have a positive charge. However Maurice has clearly answered your question and I would have upvoted but my reputation doesnt permit that. Only ions with the right injection energy will pass this region linearly when the electrical force Coulomb force dependent on ion charge and electric field strength just matches exactly the magnetic deflection Lorentz force dependent on ion velocity ion charge and magnetic field strength provided that a good homogeneity and alignment of the magnetic and electric field.
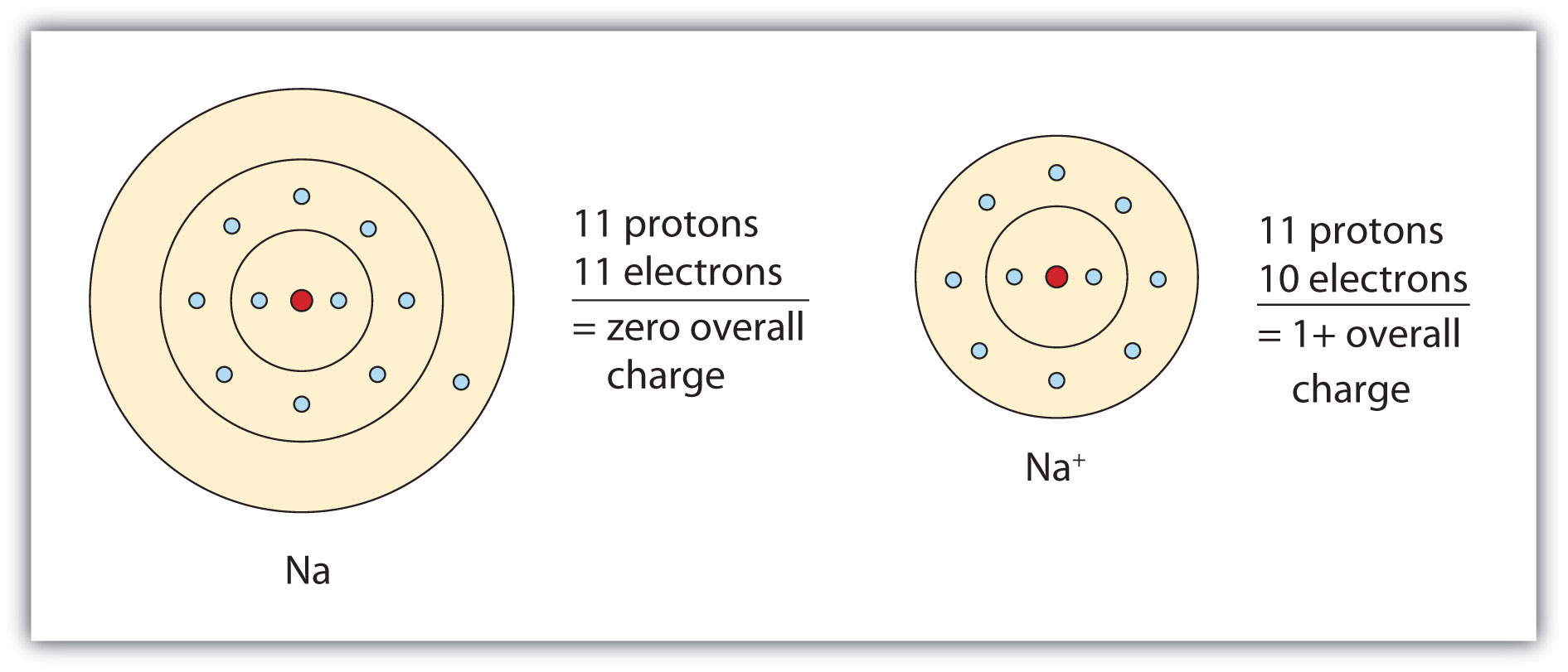
So positively charged ions move towards the negative electrode and anions move towards the positively charged electrode. When an atom gains electrons this results in a negative charge. Ionic compounds are electrically neutral because the charges of the cations and anions that make up the compound cancel each other out.
Electrons have a negative charge whereas protons have a positive charge. The number of neutrons is not a factor in whether an atom functional group or molecule is an anion. And thus the atom has no overall charge despite the.
Electrical conductivity measures the. Because of the difference in electrical potential energy between the two metals the positive and negative ions will begin to move freely But could a potato battery power for example a phone. However an electric current is not necessarily an electron current.
An electron current the flow of electrons contributes to an electric current since the electron carries negative electric charge. For example Cl - is the symbol for the chlorine. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton which is considered to be positive by convention.
Of positive and negative ions were unequal then the ionic species would have a net charge ie there would be charge separation. The flow of ions either positively or negatively charged also contributes to. As ions are moving the charge is moving.
Elementary science introduces this. It is charged because the number of electrons do not equal the number of protons in the atom or molecule. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.
Hope that helps u. Each atom lays claim to specific electrons and each electron is bound to a specific atom. You cant just have all of the electrons shift over to have a net movement of charge.
Ionic compounds are compounds made of charged particles ions. In the familiar solid conductors ie. CationIons that are positively charged because they.
In anions there are more electrons than protons. Ions are compounds that need to move around to generate electric atoms which happens easily when the solution is melted. Ionic compounds are made up of positively and.
6 1 6 1 60 6 6 0. The number of protons it has is equal to its atomic number or proton number which defines its place on the periodic table. Or by rupture of a covalent bond between two.
An ion ˈ aɪ ɒ n-ən is an atom or molecule with a net electrical charge. However if you have ions then they can serve that purpose. But electric charge is not an entity it is a property that must be carried by a charge carrier.
So ions conduct electricity in the molten form as in such a condition the intermolecular forces of attraction is very weak so flow of electrons in such compounds is very smooth and easier. Ionic conductivity is electrical conductivity due to the motion of ionic charge. Since electrons are shared in covalent bonds they cannot separate into charged ions in a solution.
Now since electrostatic forces the forces that bind the cations and anions in an ionic compound are very strong over short distances as found in a compound very large amount of work has to be done to separate the charges. IonAn atom or group of atoms bearing an electrical charge such as the sodium and chlorine atoms in a salt solution. In the case of salt for example sodium has a charge of positive one and chloride has a charge of negative one.
If you add together the charges of each particle in the atom you get. All ions are charged. They have a net electric charge and are free to move independent of one another.
When the ionic compounds are in a melted state the temperature is suitable for the electrons to charge electricity as the. Metal ions do not share electrons with one another. Negatively charged ions anions.
An atom can acquire a positive charge or a negative charge depending on whether the number of electrons in an atom is greater or less then the number of protons in the atom. In this liquid state the charged ions separate and move freely creating a current of electrical particles that conducts electricity. Like cations the charge on an anion is indicated using a superscript after a chemical formula.
Anions are ions that carry a net negative charge. An ion is defined as an atom or group of atoms where the number of electrons is not equal to the number of protons. By combination of ions with other particles.
Positively charged ions are called cations. This movement of charge produces current. The positive ions are formed by metals having lost one or more electrons.
This type of ion is called an anion. When an atom loses electrons this results in a positive charge. Consequently Ions move to respective electrodes and thus the compound is able to conduct electricity.
An ion is a charged atom or molecule.
Ch104 Chapter 3 Ions And Ionic Compounds Chemistry

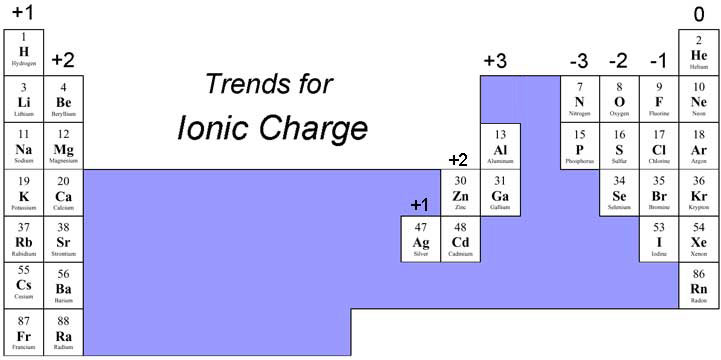
Finding The Ionic Charge Of An Element
Why Are Iron Silver And Copper Able To Form Ions With Different Charge Whilst Other Elements Cannot Quora

Molecular And Ionic Compounds Chem 1305 Introductory Chemistry
0 Comments